
Cs2 carbon disulfide molecule Royalty Free Vector Image
The first step is to sketch the Lewis structure of the CS2 molecule, to add valence electrons around the carbon atom; the second step is to add valence electrons to the two sulfur atoms, and the final step is to combine the step1 and step2 to get the CS2 Lewis Structure.

CS2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
A step-by-step explanation of how to draw the CS2 Lewis Dot Structure (Carbon disulfide).For the CS2 structure use the periodic table to find the total numbe.

CS2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
Added Jun 9, 2014 by WebTester in Chemistry This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
CS2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
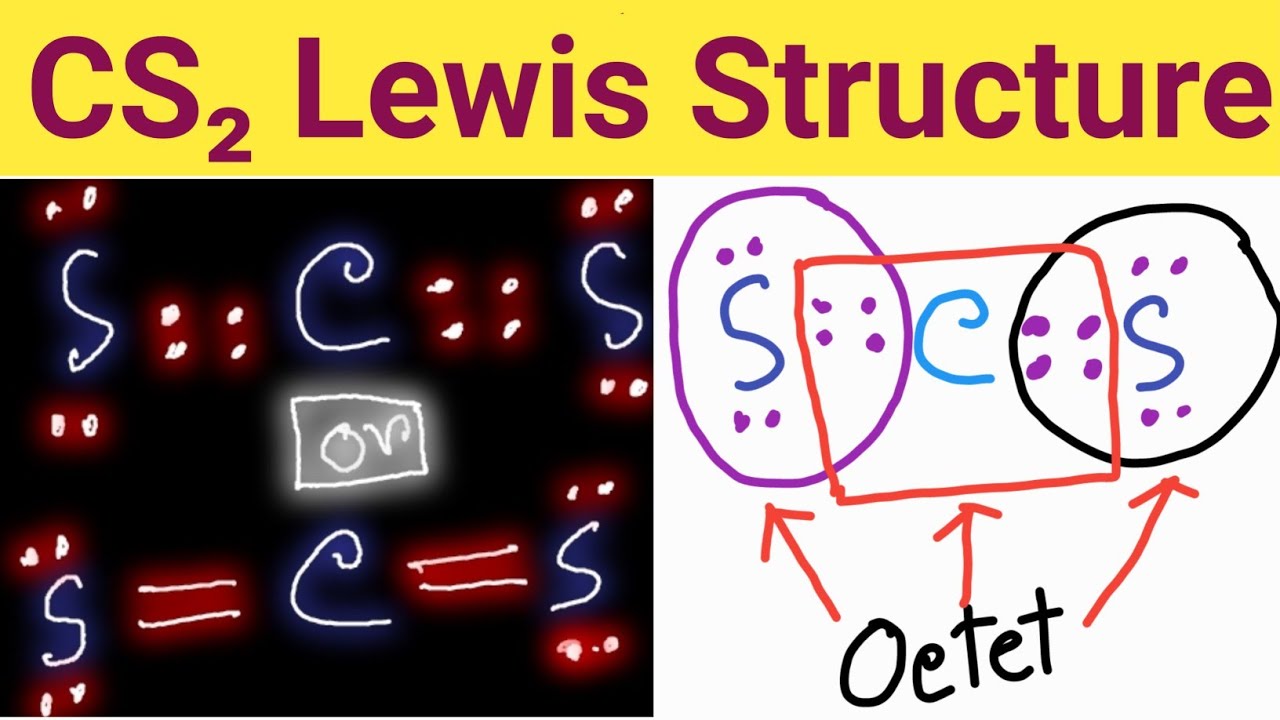
To draw the Lewis structure for CS2, follow these steps: 1. Calculate the total number of valence electrons in CS2: Carbon (C) possesses 4 valence electrons, while sulfur (S) has 6 valence electrons. As there are two sulfur atoms, the total valence electrons in CS2 sum up to 16. 2. Identify the central atom, which is the least electronegative atom.

How to draw CS2 Lewis Structure? Science Education and Tutorials
3.1: Lewis Structures. Chemical bond refers to the forces holding atoms together to form molecules and solids. This force is of an electric nature, and the attraction between electrons of one atom to the nucleus of another atom contributes to what is known as chemical bonds.
CS2 Lewis Structure Lewis Dot Structure for CS2 Carbon Disulfide Lewis Structure YouTube
The CS2 Lewis structure is a representation of the arrangement of valence electrons in the molecule. To draw the CS2 Lewis structure, one must first determine the number of valence electrons for each atom, which is 4 for carbon and 6 for sulfur.

So far, we’ve used 16 of the CS2 Lewis structure’s total 16 outermost valence shell electrons
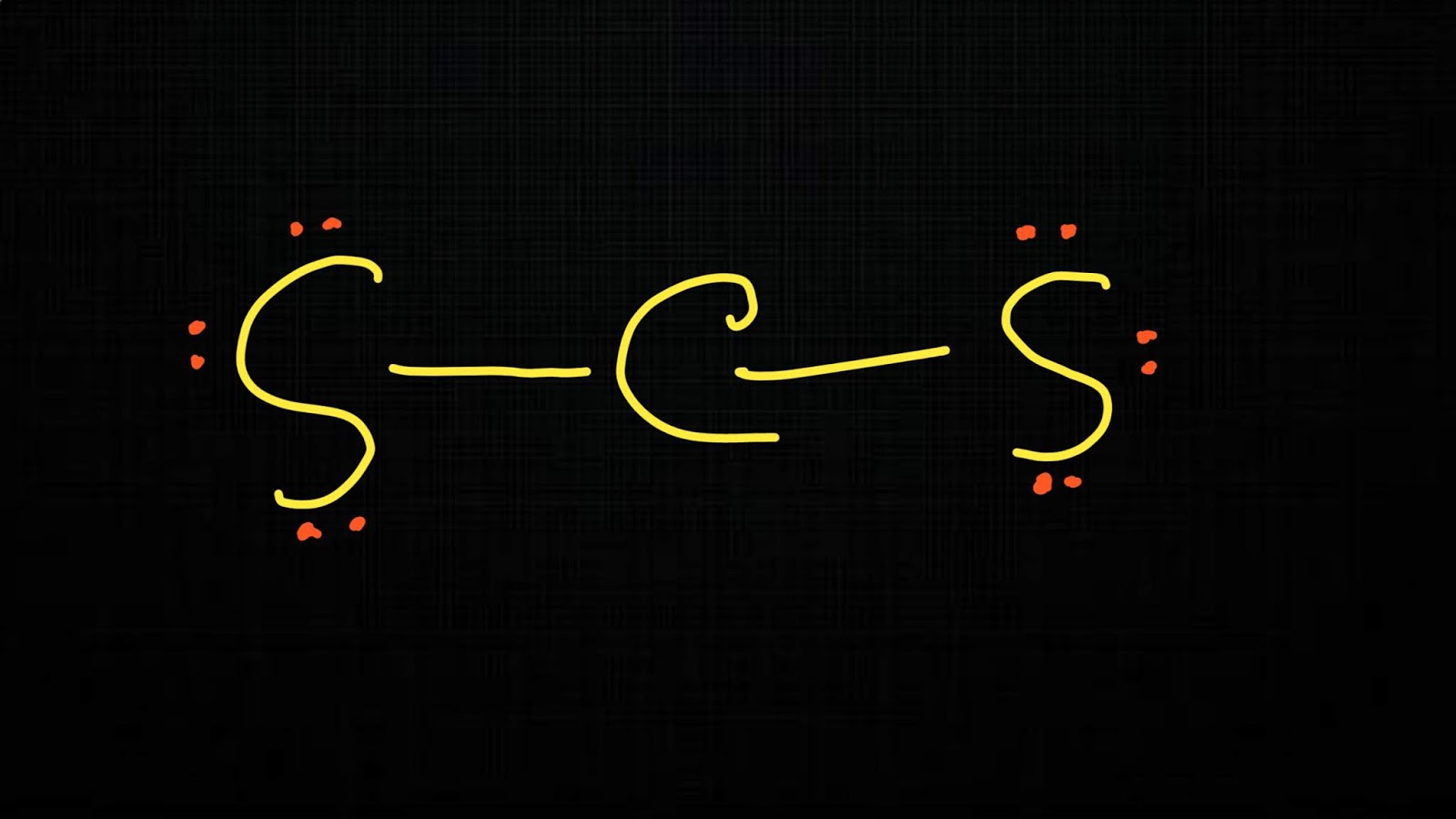
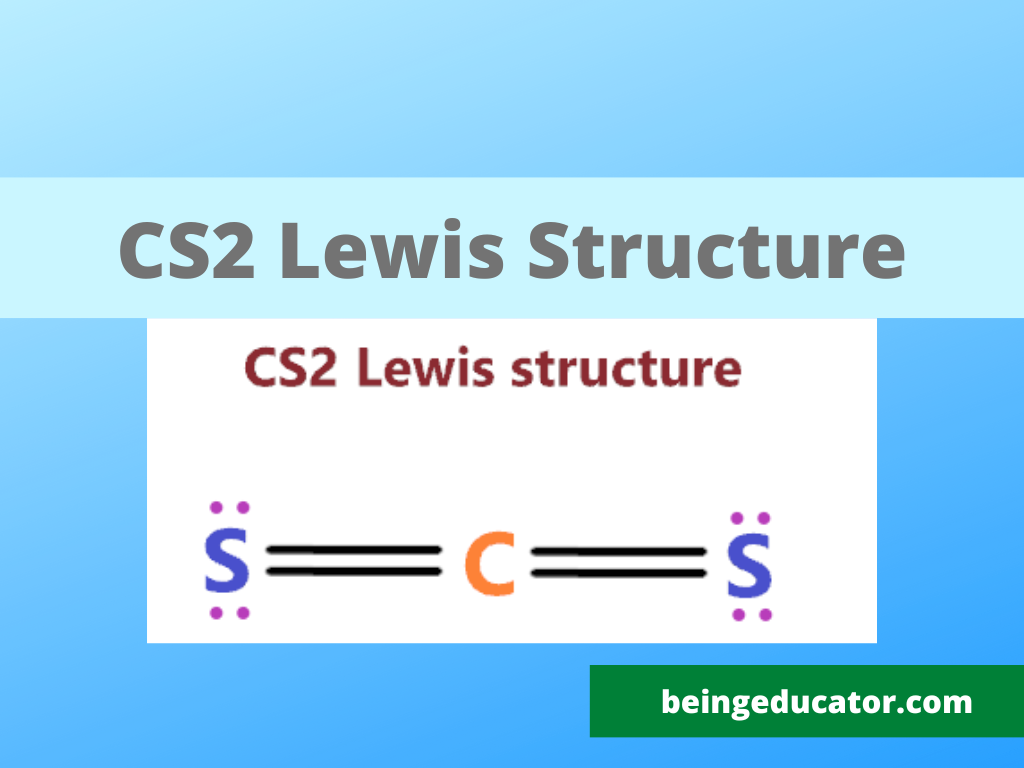
CS2 Lewis structure is made up of one carbon (C) atom, and two sulfur (S) atoms. The carbon (C) atom is kept at the central position and the Sulfur (S) atom is on either side of it in the lewis diagram. In the CS2 lewis structure, there are a total of 4 lone pairs and 2 double bonds present.
[Solved] Choose the Lewis dot formula that most accurately describes the bonding in CS2.S =C =S
Chemistry 101A Topic F: Molecular Structure 9: Basic Concepts of Covalent Bonding 9.3: Drawing Lewis Structures

CS2 Lewis Structure, Hybridization, Polarity and Molecular Shape
A quick explanation of the molecular geometry of CS2 including a description of the CS2 bond angles.Looking at the CS2 Lewis structure we can see that there.

CS2 Lewis Structure How to Draw the Lewis Structure for CS2 YouTube
CS2 Lewis Structure The first thing we need to do when drawing a Lewis structure is determine the total number of valence electrons in the molecule. Remember, valence electrons are those in the outermost principal energy level. For example: Na - 1s 2 2s 2 2p 6 3s 1, Cl - 1s 2 2s 2 2p 6 3s 2 3p 5
CS2 Lewis Structure Molecular Geometry Polarity Hybridization
For the CS2 Lewis structure, calculate the total number of valence electrons for the CS2 molecule. After determining how many valence electrons there are in CS2, place them around the central atom to complete the octets. There are 16 valence electrons for the CS2 Lewis structure.

CS2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the atoms). A dash (or line) is sometimes used to indicate a shared pair of electrons: A single shared pair of electrons is called a single bond.

Cs2 Lewis Structure / Identify the geometry of if5 using vsepr theory. Anabelfl
6 steps of CS2 Lewis structure: Step 1: Total number of valence electrons: Step 2: Determine the central metal atom: Step 3: Connect the atoms with dots: Step 4: Distribution of remaining valence electrons: Step 5: Check if all atoms have an octet: Step 6: Formal charge: Synthesis/Production: Direct Synthesis:

CS2 Molecular Geometry / Shape and Bond Angles YouTube
A step-by-step explanation of how to draw the CS2 Lewis Dot Structure (Carbon disulfide).For the CS2 structure use the periodic table to find the total numbe.

How do you draw the Lewis structure of CS2 (Carbon disulfide) YouTube
In Lewis structure of CS2 molecule, there are 16 valence electrons, out of which four valence electrons are of Carbon, and six valence electrons are from each sulfur molecule. Carbon is the least electronegative molecule and thus comes in the center. These two sulfur molecules form double bonds with this Carbon molecule to complete Carbon's octet.

CS2 Molecular Geometry Science Education and Tutorials
Lewis structure of CS2 (or Carbon Disulfide) contains two double bonds between the Carbon (C) atom and each Sulfur (S) atom. The Carbon atom (C) is at the center and it is surrounded by 2 Sulfur atoms (S). The Carbon atom does not have a lone pair while both the Sulfur atoms have 2 lone pairs.